The Cancer Survivors Network (CSN) is a peer support community for cancer patients, survivors, caregivers, families, and friends! CSN is a safe place to connect with others who share your interests and experiences.
More articles

The CRC breakup? http://www.mdpi.com/1422-0067/19/9/2577/htm
Colon cancer (CC) and rectal cancer (RC) are synonymously called colorectal cancer (CRC). Based on our experience in basic and clinical research as well as routine work in the field, the term CRC should be abandoned. . . we conclude that CC is not the same as RC. The term “CRC” should no longer be used as a single entity in basic and clinical research as well as other areas of classification.
Move over platinum, here comes titanium (abstract only): https://www.ncbi.nlm.nih.gov/pubmed/30203598
Due to the toxicity of platinum compounds used in the clinic as anticancer chemotherapies, the safe titanium serves as an attractive alternative. Lately, we introduced a new family of Ti complexes based on readily available phenolato ligands, demonstrating an incredibly high hydrolytic stability, with the lead compound phenolaTi demonstrating a wide cytotoxic activity towards the NCI-60 panel of NIH human cancer cell lines, with an average GI50 of 4.7±2 µM. Herein, we evaluated in vivo: (a) the safety, and (b) the growth inhibitory (efficacy) of this compound. PhenolaTi was effective in vivo against colon (CT-26) and lung (LLC-1) murine cell lines in syngeneic hosts and towards a human colon cancer (HT-29) cell line in immune deficient (Nude) mice, with an efficacy similar to that of known chemotherapy. Notably, no clinical signs of toxicity were observed in the treated mice; namely, no effect on body weight, spleen weight or kidney function, unlike observed with the positive control Pt drugs. Studies of combinations of PhenolaTi and Pt drugs evinced that similar efficacy with reduced toxicity may be achieved, which is highly valuable for medicinal applications.
Eat your watermelon: https://www.ncbi.nlm.nih.gov/pubmed/30207495
Diets high in fruits and vegetables may help prevent colorectal cancer (CRC). Watermelon consumption may reduce CRC risk due to its concentration of l-citrulline and its role in endothelial nitric oxide (NO) production. Research suggests that increased NO levels have tumoricidal effects. . . These results suggest that watermelon powder or l-arginine supplementation may reduce the risk of colon cancer by suppressing ACF formation through lowering oxidative DNA damage and inflammation, modulating DNA repair enzyme expression, and/or enhancing NO production.
Berberine (available as a nutritional supplement, this is a new one on me): http://www.mdpi.com/1420-3049/23/9/2298/htm
Berberine (BBR) has been extensively reported to inhibit colorectal cancer (CRC) development, though its bioavailability is poor. Nowadays, an increasing number of studies have shown that BBR significantly accumulates in the intestines and could regulate gut microbiota in obesity. . . Conclusions: BBR significantly alleviated the development of CRC in Apc min/+ mice fed with HFD and restored the enteric microbiome community.
Another (abstract only): https://www.ncbi.nlm.nih.gov/pubmed/30125974 Berberine (BBR) is a benzyl tetra isoquinoline alkaloid exracted from several plants. BBR is nontoxic to human normal cells, but suppresses the growth of different tumor cells: melanoma, epidermoid carcinoma, hepatoma, oral carcinoma, glioblastoma, prostatic carcinoma, and gastric carcinoma. In particular, BBR seems to suppress the proliferation of gastrointestinal cancers in a number of preclinical models
Magnolia Berry (five flavor berry): https://www.ncbi.nlm.nih.gov/pubmed/30210348
Gomisin A (G.A) is a dietary lignan compound from Schisandra chinensis. In this study, the effect of G.A on the proliferation and metastasis of colorectal cancer (CRC) cells was investigated using several CRC cell lines and a lung metastasis mouse model. . . G.A ameliorated lung metastasis of CRC cells by decreasing cell survival and metastatic abilities of CRC cells. Thus, G.A might be a potential novel therapeutic agent for metastatic CRC.
The mircobiome (eat your yogurt): http://www.mdpi.com/2305-6320/5/3/101/htm
Studies so far have suggested that restoring function to the microbiome can have beneficial effects in the prevention of cancer and in improving the effectiveness and safety of cancer treatment. As we have seen, administration of certain Lactobacillus and Bifidobacterium strains is associated with biochemical and histologic changes that may decrease the risk of developing malignancy [50,51]. Other Lactobacillus [40], Bifidobacterium [17], and Bacteroides [16] strains seem to play critical roles in the effectiveness of certain cytotoxic and immune therapies. Nonetheless, the bulk of research in these topics has been in animal models. Data from human research, particularly in clinical trials evaluating whether probiotics or synbiotics can alleviate the adverse effects of various cancer therapies, has so far shown mixed results.
Put some cinnamon on your yogurt: https://www.ncbi.nlm.nih.gov/pubmed/30209760
There is a lot of evidence suggesting that a small subset of cancer cells resistant to conventional chemotherapy and radiotherapy and known as cancer stem cells (CSCs) is responsible for promoting metastasis and cancer relapse. Therefore, targeting and eliminating the CSCs could lead to higher survival rates and a better quality of life. In comparison with conventional chemical drugs that may not be effective against CSCs, phytochemicals are strong anti-CSCs agents. The current study examines the effect of 5-fluorouracil plus oxaliplatin (FOLFOX) as a common chemotherapy drug on colorectal cancer as well as the influence of Cinnamic acid (CINN) as a plant-derived phytochemical on colon cancer stem-like cells in HT-29 adenocarcinoma cell line. . . The FOLFOX and CINN decreased cell viability in certain drug concentrations: IC50 = 5,40 μM oxaliplatin +220 μM 5-fluorouracil, and 13,50 mM for CINN. The CSC-associated markers (OCT4, NANOG, ABCB1, and ALDH1A) and the proportion of cancer stem-like cells (SP cells, CD44, and CD133 positive cells) were downregulated following the treatment of HT-29 adenocarcinoma cell line with IC50 concentrations of FOLFOX and CINN.
And eat some chickpeas (actually this is getting fatiguing, eat a wide variety of healthful, whole foods, and you will probably optimize your odds): https://www.ncbi.nlm.nih.gov/pubmed/30211662
Dietary strategies: https://cancerci.biomedcentral.com/articles/10.1186/s12935-018-0631-y
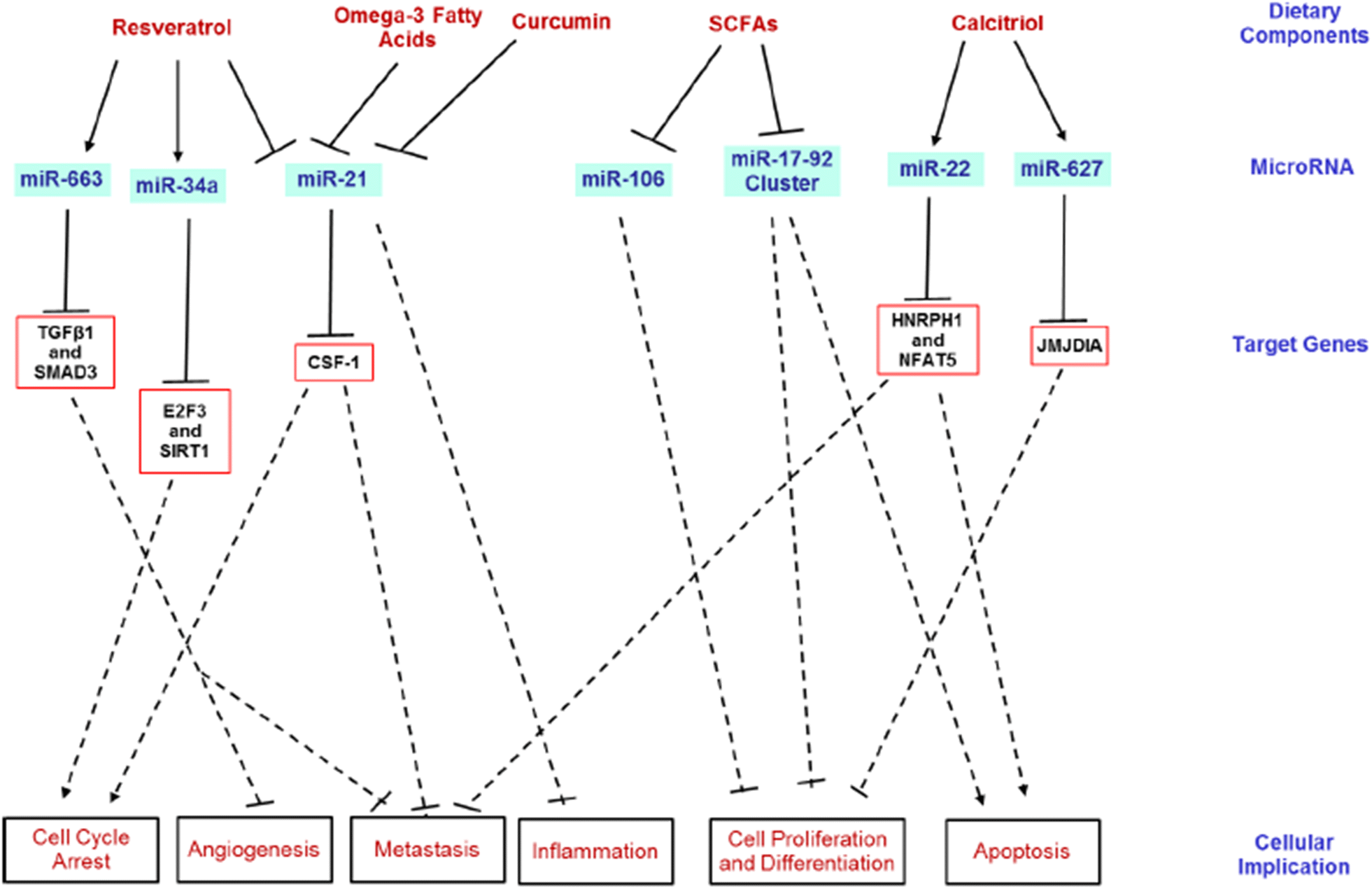
[S]trong evidence continues to show that certain dietary components possess cancer-protective capabilities, including therapeutic and chemopreventive properties. These dietary factors may play a role in several stages of carcinogenesis, such as cell-cycle modulation, inflammation, apoptosis, DNA repair and angiogenesis [111]. miRNAs are intrinsically involved in similar stages of carcinogenesis, which widens the understanding between miRNAs and certain dietary components (Fig. 4). Certain dietary components of plant origin may be less bio-available and thus, escape digestion into the large intestine. Therefore, these bioactive components may then play a role in modulating CRC.
Microsatelite stability/instability and survival: https://www.ncbi.nlm.nih.gov/pubmed/30216221
Microsatellite instability was independently associated with a reduction in pCR for locally advanced rectal cancer after neoadjuvant chemoradiation in this NCDB-based analysis.
Vitamin C: https://www.ncbi.nlm.nih.gov/pubmed/30214687
The dual administration of demethylating agents and vitamin C to colorectal cancer patients, a demographic in which vitamin C deficiencies are common, may improve responses to epigenetic therapies.
Vitamin C again: https://www.ncbi.nlm.nih.gov/pubmed/30217071
At the clinical level, [Vitamin C]AA is associated with tumour regression in advanced disease and improved tolerability and side effects of standard therapy. Based on these findings, we conclude that further clinical trials are needed on a larger scale to examine the therapeutic benefits of AA in colon cancer.
Aspirin: https://www.ncbi.nlm.nih.gov/pubmed/30214631
We found that aspirin induces apoptosis in enriched colorectal CSCs, inhibits tumor progression, and enhances the anti-neoplastic effects of chemotherapeutic agents. Furthermore, aspirin directly interacts with p300 in the nucleus, promotes H3K9 acetylation, activates FasL expression, and induces apoptosis in colorectal CSCs. Notably, these effects of aspirin are absent in non-CSCs since H3K9 is hypermethylated in non-CSCs and the effects are not induced by other NSAIDs. In addition, aspirin can suppress oxaliplatin-enriched CSCs and serve as an adjuvant therapy. Conclusions: Taken together, we revealed a unique epigenetic and cox-independent pathway (p300-AcH3K9-FasL axis) by which aspirin eliminates colorectal CSCs. These findings establish an innovative framework of the therapeutic significance of aspirin.
Curcumin and oligomeric proanthocyanidins (such as grape seed extract): https://www.ncbi.nlm.nih.gov/pubmed/30218018
We validated genes belonging to these pathways, such as HSPA5, SEC61B, G6PD, HMOX1 and PDE3B to be cooperatively modulated by the OPCs-curcumin combination. We further confirmed that the OPCs-curcumin combination more potently suppresses colorectal carcinogenesis and modulated expression of genes identified by RNA-sequencing in mice xenografts and in colorectal cancer patient-derived organoids. Overall, by delineating the cooperative mechanisms of action of OPCs and curcumin, we make a case for the clinical co-administration of curcumin and OPCs as a treatment therapy for patients with colorectal cancer.
Omega 3's: https://www.tandfonline.com/doi/abs/10.1080/14737140.2018.1524299?journalCode=iery20
Although inflammation is defensive and healing process that maintains organ homeostasis, unresolved inflammation can lead to diseases. Polyunsaturated fatty acids (PUFAs), especially n-6 PUFAs abundant in Western diet, are precursors of pro-inflammatory mediators, whereas n-3 PUFAs possess anti-inflammatory properties.
Phytochemicals: http://www.mdpi.com/1422-0067/19/9/2729/htm
Several studies have reported that many phytochemicals have anti-inflammatory, anti-oxidation, chemopreventive and anti-cancer properties [13,14]. Recently, some phytochemicals can inhibit pattern recognition receptor (PRR) activation by targeting the receptor or the downstream signaling molecules [15]. In this review, we will discuss the different functions of TLR4 in cancer progression and inflammation. Furthermore, we also summarize and discusses the recent findings of curcumin, 6-gingerol, 6-shogaol, 1-dehydro-10-gingerdione, EGCG, luteolin, quercetin, resveratrol, caffeic acid phenethyl ester, xanthohumol, genistein, berberine, and sulforaphane in inhibiting the activation of TLR4.
Comments
Discussion Boards
- All Discussion Boards
- 6 Cancer Survivors Network Information
- 6 Welcome to CSN
- 122.6K Cancer specific
- 2.8K Anal Cancer
- 457 Bladder Cancer
- 312 Bone Cancers
- 1.7K Brain Cancer
- 28.6K Breast Cancer
- 407 Childhood Cancers
- 28K Colorectal Cancer
- 4.6K Esophageal Cancer
- 1.2K Gynecological Cancers (other than ovarian and uterine)
- 13.1K Head and Neck Cancer
- 6.4K Kidney Cancer
- 683 Leukemia
- 804 Liver Cancer
- 4.2K Lung Cancer
- 5.1K Lymphoma (Hodgkin and Non-Hodgkin)
- 242 Multiple Myeloma
- 7.2K Ovarian Cancer
- 70 Pancreatic Cancer
- 493 Peritoneal Cancer
- 5.6K Prostate Cancer
- 1.2K Rare and Other Cancers
- 544 Sarcoma
- 744 Skin Cancer
- 661 Stomach Cancer
- 193 Testicular Cancer
- 1.5K Thyroid Cancer
- 5.9K Uterine/Endometrial Cancer
- 6.4K Lifestyle Discussion Boards
